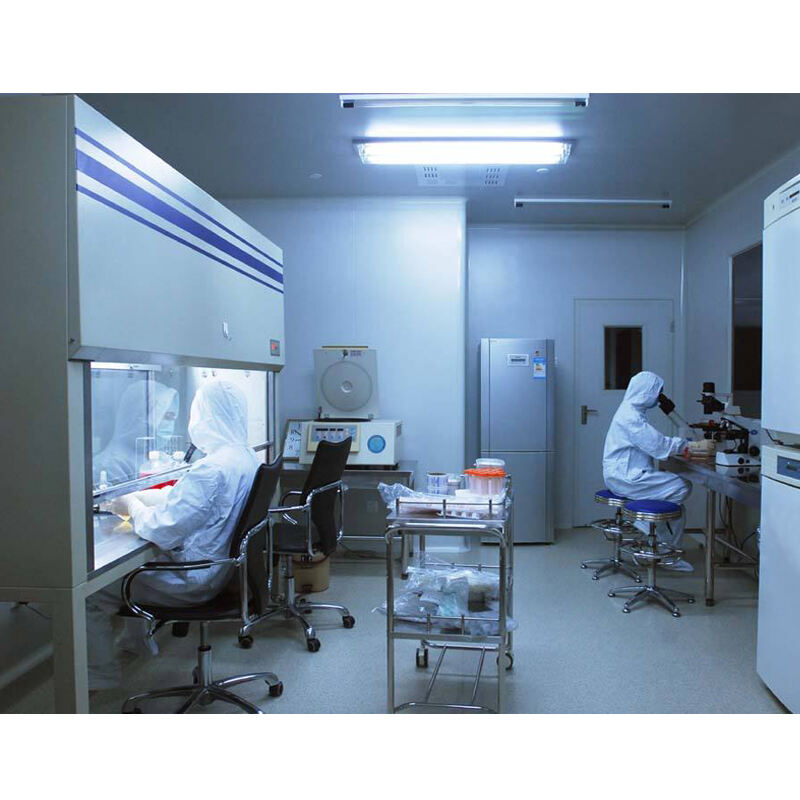
gmp room
A GMP (Good Manufacturing Practice) room represents a meticulously controlled environment designed to ensure the highest standards of cleanliness and contamination control in pharmaceutical, biotechnology, and medical device manufacturing. These specialized facilities incorporate advanced HVAC systems with HEPA filtration, maintaining precise temperature, humidity, and air pressure differentials. The room's construction features seamless walls, floors, and ceilings with rounded corners to prevent particle accumulation and facilitate thorough cleaning. Advanced monitoring systems continuously track environmental parameters, while airlocks and gowning rooms serve as critical transition zones. The space is equipped with validated cleaning protocols, decontamination systems, and specialized furniture designed for cleanroom use. Material flow and personnel movement are carefully controlled through designated pathways and strict operational procedures. These rooms support various manufacturing processes, quality control testing, and research activities while ensuring compliance with regulatory requirements and maintaining product integrity throughout the production cycle.