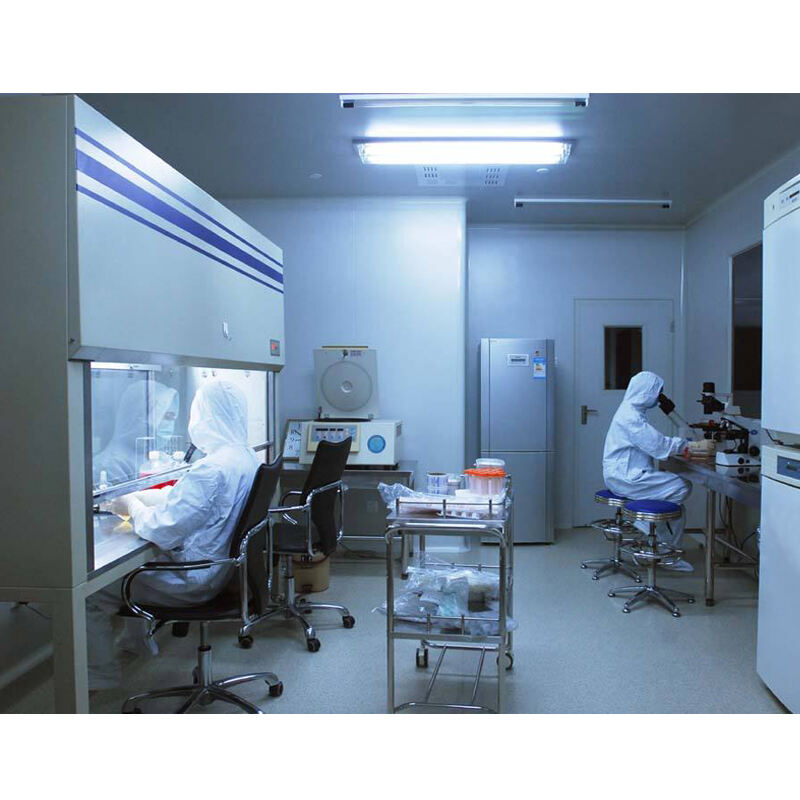
gmp clean room
A GMP clean room represents a highly controlled environment designed to maintain specific levels of cleanliness and environmental control, essential for pharmaceutical, medical device, and biotechnology manufacturing. These specialized facilities incorporate advanced filtration systems, precise temperature and humidity controls, and sophisticated air handling units to maintain optimal conditions. The room's design includes seamless walls, floors, and ceilings constructed from non-porous materials that resist microbial growth and facilitate thorough cleaning. Multiple pressure differentials between adjoining spaces prevent cross-contamination, while HEPA filtration systems remove airborne particles down to 0.3 microns. The facility's monitoring systems continuously track critical parameters including particle counts, air pressure, temperature, and relative humidity, ensuring compliance with regulatory standards. Standard operating procedures govern all activities within the clean room, from gowning protocols to cleaning schedules, maintaining the stringent cleanliness requirements necessary for GMP certification. These facilities are classified according to ISO standards, with classifications ranging from ISO 1 to ISO 9, depending on the maximum allowable particles per cubic meter of air.